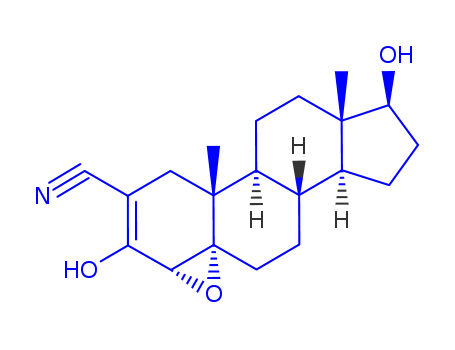
Trilostane
Trilostane
Trilostane Good Supplier In Bulk Supply High Purity 13647-35-3
- Molecular Formula:C20H27NO3
- Molecular Weight:329.439
- Appearance/Colour:tan crystals
- Vapor Pressure:5.39E-12mmHg at 25°C
- Melting Point:264 °C
- Refractive Index:1.61
- Boiling Point:497.8 °C at 760 mmHg
- PKA:8.57±0.70(Predicted)
- Flash Point:254.8 °C
- PSA:76.78000
- Density:1.25 g/cm3
- LogP:3.46688
Trilostane(Cas 13647-35-3) Usage
|
treatment of Cushing's syndrome |
Trilostane is an inhibitor of 3β-hydroxysteroid dehydrogenase used in the treatment of Cushing's syndrome and primary hyperaldosteronism. These are both disorders where excess amounts of corticosteroid hormones are produced in the body. Corticosteroids are essential for the body to make use of carbohydrates, fats and proteins and for a normal response to stress. They are also necessary for the regulation of salt and water balance in the body. Trilostane helps prevent the production of corticosteroids, controlling the symptoms associated with these disorders. Trilostane can also be useful in the treatment of breast cancer that has relapsed in women who have gone through menopause. |
|
Uses in dogs |
Trilostane was withdrawn from the United States market in April 1994. However, it was approved in 2008 for the treatment of Cushing's disease (hyperandrenocorticism) in dogs. It is also the first drug approved to treat both pituitary-and adrenal-dependent Cushing's in dogs. 1. has kidney or liver disease. 2. takes certain medications used to treat heart disease. 3. pregnant. 4. taking other medicines, including those available to buy without a prescription, herbal or complementary medicines. 5. had an allergic reaction to this or any other medicine. |
|
Indications |
Trilostane can inhibit 3β-dehydrogenase in the synthesis of corticosteroids, and decrease the synthesis of cortisol and aldosterone. It also can be used in the treatment of Cushing's syndrome (hypercortisolism) and primary aldosteronism. But the efficacy to treat Cushing's syndrome (hypercortisolism) is not as metyrapone. This product also has a significant role in lowering blood testosterone levels, which may be related with that its synthesis is inhibited. |
|
Treatment history |
Trilostane, that is 4α, 5α-epoxy-2-cyano-3,17β-diol, can reversibly inhibit 3β-hydroxy steroid dehydrogenase and △5, 4-isomerase in the adrenal cortex to block the biosynthesis of mineralocorticoid and glucocorticoid. In 1980, it was approved for the treatment of hyperaldosteronism and cortisol excess disease in the United Kingdom. In 1985, it was approved for the treatment of Cushing's syndrome in the United States, which is adrenal cortex hyperactivity disorder. In view of the older animals, especially older dogs are susceptible to Cushing's syndrome. Trilostane can relieve the disease symptoms of more than 90% of dogs, and improve their quality of life. So the drug has also been granted for the treatment of animals in the United Kingdom. Trilostane itself has no hormonal activity. Its side effects is less, and its safety and tolerability are also particularly good. The above information is edited by the lookchem of Ge Qian. |
|
Mechanism |
Breast cancer is one of hormone-dependent tumors. And estrogen is the main driving factor of the growth of breast cancer cells. Thus, one of the modern treatment of breast cancer is the major target of estrogen through inhibiting the production of estrogen or block the action of estrogen or estrogen receptor in its site of action. It has been known that estrogen receptor is only a single receptor, but recent studies have identified that there are at least two subtypes α and β. Estrogen uniting with α-estrogen can stimulate cell growth, but it uniting with β-estrogen receptor can cut α-estrogen receptors and slow the rate of cell proliferation. Research revealed that the trilostane cannot only lower the production of estrogen, but also can modulate the binding of estrogen receptors on different subtypes. And meanwhile the dual effect of α-estrogen receptor inhibitors and β-estrogen receptor appears. Finally it can block and alter the negative effect of estrogen on cancer cells. The unique mode of action of trilostane can not only set it apart from other existing anti-estrogen drugs, but also be the pharmacological basis of that it is still highly effective on breast cancer in treatment failure with other anti-estrogen therapy or resistant breast cancer. |
|
The treatment of advanced breast cancer |
Breast cancer is the most common type of tumor in women. Currently, for postmenopausal patients with hormone receptor-positive or unknown, clinical treatments unanimously recommend to be the preferred antiestrogen therapy, and the role of this therapy in delaying disease progression and improving survival time also has been affirmed and confirmed in numerous studies of breast cancer. Trilostane (trade name Modrenal) developed by the biotechnology company has already been on the market. It can be used for the treatment of postmenopausal women that hormone-selective cancer has spread outside the breast. The pharmaceutical uses two ways to slow down the disease progression. For hormone-sensitive breast cancer, estrogen promotes the growth of cancer cells by acting to the two receptors. Estrogen receptor α is cancer accelerator, and estrogen receptor β is the brake. Modrenal strengthens estrogen adsorption to the estrogen receptor β, and weakens the adsorption to estrogen receptor α. Meanwhile it also acts on another loci AP1in cell DNA to reduce cell proliferation. |
|
Manufacturing Process |
(A)17β-acetoxy-4α,5α-epoxyandrostano[2,3-d]isoxazole, melting point 228.6°C to 229.8°C (corrected) recrystallized from a benzene-methanol mixture, [α]D25 = +76.5°C (1% in chloroform), was prepared by treating 17β- acetoxy-4-androsteno[2,3-d] isoxazole with maleic anhydride and hydrogen peroxide in methylene dichloride solution. (B)2α-cyano-4α,5α-epoxandrostan-17β-ol-3-one was prepared by treating 17β-acetoxy-4α,5α-epoxyandrostano[2,3-d] isoxazole with sodium methoxide, and was obtained in the form of tan crystals, melting point 257.8°C to 270.0°C (decomposition) (corrected) when recrystallized from a pyridine_x0002_dioxane mixture. |
|
Brand name |
Modrastane (Bioenvision). |
|
Therapeutic Function |
Corticosteroid antagonist |
|
General Description |
Trilostane inhibits the production of adrenal steroids, such as cortisol and aldosterone. It is used to treat aldosteronism. |
|
Biochem/physiol Actions |
Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase (3-β-HSD or delta 5-delta 4-isomerase), an essential enzyme for the biosynthesis of all classes of hormonal steroids. It has been used in the treatment of Cushing′s syndrome for stopping the production of cortisol, and is currently approved for dogs in the US, but is still a human drug in the UK and other countries. It is being investigated as a possible treatment for both breast cancer and prostate cancer to prevent the synthesis of estrogens and androgens from endogenous precursors. It has also been used to inhibit endogenous production of progesterone in research studies. |
|
Veterinary Drugs and Treatments |
Trilostane may be useful for treating pituitary-dependent hyperadrenocorticism or adrenal dependent hyperadrenocorticism in dogs, feline pituitary-dependent hyperadrenocorticism, and equine hyperadrenocorticism (HAC). It may also be useful in treating Pomeranians with Alopecia X and Alaskan malamutes with adultonset alopecia. |
InChI:InChI=1/C20H27NO3/c1-18-7-6-14-12(13(18)3-4-15(18)22)5-8-20-17(24-20)16(23)11(10-21)9-19(14,20)2/h12-15,17,22-23H,3-9H2,1-2H3/t12-,13-,14-,15-,17+,18-,19+,20+/m0/s1
13647-35-3 Relevant articles
METHOD FOR THE PREPARATION OF TRILOSTANE
-
Page/Page column 7-8, (2008/06/13)
The present invention relates to a metho...
13647-35-3 Process route
-
![4α,5-epoxy-(5α)-androstano[2,3-<i>d</i>]isoxazol-17β-ol](/upload/2024/8/408bd245-5164-4b2a-8f3d-a3aa10ee3c79.png)
-
20051-76-7
4α,5-epoxy-(5α)-androstano[2,3-d]isoxazol-17β-ol

-
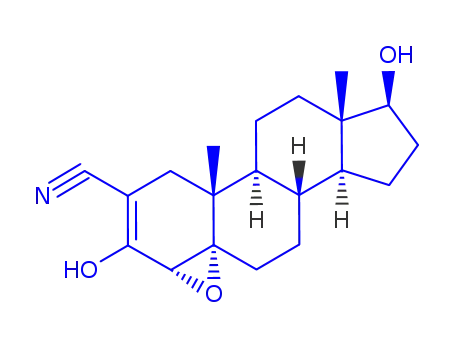
-
13647-35-3
Trilostane
| Conditions | Yield |
|---|---|
|
4α,5-epoxy-(5α)-androstano[2,3-d]isoxazol-17β-ol;
With
sodium hydroxide;
In
methanol;
at 40 - 45 ℃;
for 2h;
With
acetic acid;
In
methanol; water;
at 40 - 45 ℃;
for 2h;
|
95% |
13647-35-3 Upstream products
-
20051-76-7
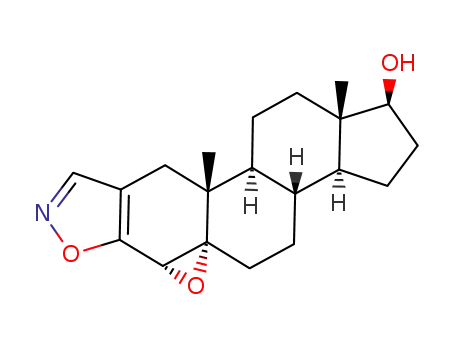
4α,5-epoxy-(5α)-androstano[2,3-d]isoxazol-17β-ol